Laser Equipment Review
There are many types of laser sources available. This will focus solely on Infrared fiber laser technology.
What is IR?
Infrared light exists in the electromagnetic spectrum. The visible light region ends at 700 nm IR exists from 700 nm to approximately 1 mm wavelength. As a photon IR light propagates both energy and momentum. For discussions of laser technology, including fiber laser technology, it is useful to be able to discuss both.
A Photon is loosely defined as a particle of light. Light generally behaves as a wave but can also act as a particle. This is known in quantum physics as “wave-particle” duality.
This model was generated from the combined work of Gilbert N. Lewis, Max Planck and Albert Einstein.
Being outside of the visible light range this radiation cannot be “seen” by humans. Even low levels of IR can cause blinding effects.
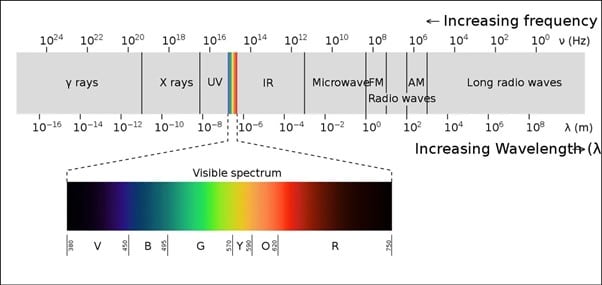
Wavelength
When we speak of colors of light we are referring to differing bands of wavelength. Yellow light has a smaller wavelength and higher frequency than red light for example. The diagram below is an illustration of how this applies to visible light.
IR radiation is light outside of the visible spectrum that begins immediately after the red range continuing on with longer wavelengths.
UV radiation is the light outside of the visible spectrum that ends immediately before the purple side preceding it with higher frequency and shorter wavelengths.
Laser Light
The term “laser” is an acronym which stands for Light Amplification by Stimulated Emission of Radiation. To fully understand this it’s important to understand the relationship between atoms and photons.
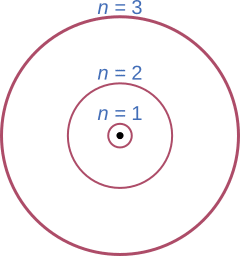
The Bohr model of the atom had a central body known as the nucleus which is surrounded by electrons orbiting it. These electrons can only exist at discreet distances from the nucleus. These distances are referred to as “energy levels”. Due to the law of conservation of energy electrons cannot simply “jump” between without releasing or absorbing energy.
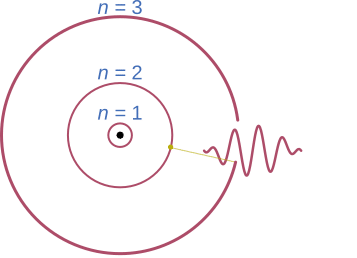
This release is what conserves the total amount of energy therefore not breaking the aforementioned law. The energy of the photon and wavelength are correlated to the distance between energy levels.
This effect works in the reverse also. An atom with its electron in the low energy state can absorb a photon and lift the electron to the outer layer. When this happens the electron is described as being in an “excited” state.
When this drop occurs unprompted it is called spontaneous emission. Stimulated emission on the other hand is where this emission is prompted. This is done by introducing more photons of the same wavelength. The technical term for this process is “pumping”. The environment in which this occurs is called the laser medium (more on this later). As photons are introduced to the laser medium several of them will lift electrons to their excited state.
However, when a photon interacts with an already excited electron it can knock it from its energy level releasing a new photon with it. The new photon will be “in-phase” with the original.
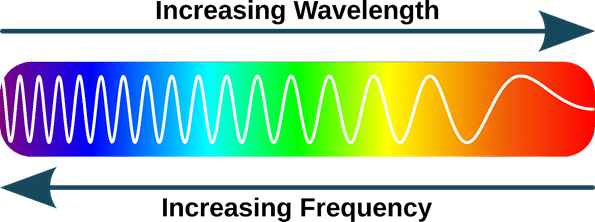
The image below illustrates how in-phase waves interact.
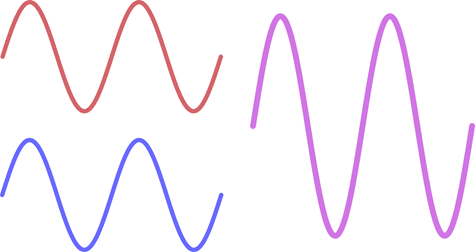
The red and blue are perfectly aligned peak to peak and thus the resulting wave will be the cumulative height of both together.
The image below illustrates how out-of-phase waves interact.
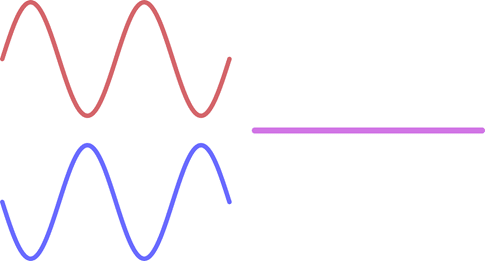
The red and blue are perfectly aligned peak to trough and thus the resulting wave will be the cumulative height of both together. In this case will effectively cancel each other out.
As mentioned before when stimulated emission occurs the photons are in phase. This means that the power will be much greater than randomly phased light but also the power output will be much more consistent.
Back to the photon generation stage. After “knocking” there will be a pair of photons moving in the medium. Eventually these will knock another electron down releasing a third photon. This compounding effect is how laser light is generated.
Light that is monochromatic (of a single wavelength) and in phase is known as coherent light.
Laser Medium
The medium in which a laser is generated will determine the wavelength (λ) emitted. We can compare fiber laser vs CO2. CO2 for example will generate IR photons with λ ≈ 10,640nm. Whereas Fiber lasers specifically Nd:YAG will generate with λ ≈ 1,064nm. These are generally referred to as “far IR” and “near IR” respectively. Below is a table illustrating some different mediums and their respective wavelengths.
| Name | Medium | λ Wavelength (nm) | Bandina |
|---|---|---|---|
| Carbon Dioxide | CO2 | 10640 | Far IR |
| Helium-Neon | He-Ne | 633 | Visible Red |
| Neodymium YAG | Nd:Yag | 1064 | Near IR |
Fiber Laser vs CO2
Gas Lasers
Typically these are cheaper options where the laser medium is CO2 or helium-neon (He-Ne).
Fiber Lasers
Fiber laser technology uses a fiber optic cable to generate and transfer laser light. Fiber optic cables by themselves cannot create laser beams. They must first be “doped” with rare elements. The most commonly used are erbium, ytterbium, neodymium, thulium, praseodymium, holmium or dysprosium.
Class Summary
| Class | Information | Example |
|---|---|---|
| CLASS 1 | Safe. | CD/DVD drive where the laser source is fully enclosed within the casing |
| CLASS 1M | Safe provided optical instruments are not used. | |
| CLASS 2 | Visible lasers. Safe for accidental exposure (< 0.25 s). | |
| CLASS 2M | Visible lasers. Safe for accidental exposure (< 0.25 s) providing optical instruments* are not used. | |
| CLASS 3R | Not safe. Low risk. | |
| CLASS 3B | Hazardous. Viewing of diffuse reflection** is safe. | |
| CLASS 4 | Hazardous. Viewing of diffuse reflection is also hazardous. Fire risk. | Open laser marking system. High likelihood of light escaping to the surroundings. |
